|
C. Loring Brace University of Michigan .
The first Europeans to write about their impressions of the newly-encountered
inhabitants of the Western Hemisphere were struck by the lack of marked visible
differences between them. The phrase written by Antonio de Ulloa in 1772
declared that, “Visto un Indio de qualquier region, se puede decir que
se hen visto todos in quanta al color y contextura” (quoted in Stewart
and Newman 1951: 19). This got shortened in colloquial American English to
read, “An Indian’s an Indian, if you’ve seen one, you’ve
seen them all.” As more than one observer has noted, this may be something
of an overstatement but one that has a germ of truth to it (Stewart 1960:259).
Certainly there are differences between the Native Americans from Alaska
and Ecuador, but that degree of difference is dwarfed by the distinctions
of comparably distant peoples in the Old World. The contrast in appearance
between the inhabitants of Oslo at sixty degrees north latitude and the natives
of Nairobi on the equator is orders of magnitude greater than the contrast
between the sixty degree north latitude Inuits of Nome Alaska and the equatorial
residents of Quito in Ecuador. If we had no more evidence than this, we would
have to conclude that the inhabitants of the Western Hemisphere had been
in place for only the smallest fraction of time that the inhabitants of the
Old World had been living at roughly the same latitudes where they are found
today. In addition, we could guess that the New World denizens had all descended
from people in a relatively restricted portion of the Old World. Sheer
geographical propinquity makes Northeast Asia the logical can
Fig.1: Ainu man and women at Piratori, on the Island of Yezo (photo: Bishop
1925, Smithsonian Ann. Rep.).didate as was
realized by people such as Fray Jose de Acosta in the late sixteenth century
and Thomas Jefferson in the 18th (Acosta 1589 [1880]; Jefferson 1787).
When eastern Asia is mentioned, the people who immediately come to mind are
the Chinese and those of similar appearance in Mongolia, Korea, and Japan
as well as farther down in Southeast Asia. There is an immediate credibility
problem in suggesting Chinese form as a model for the source of the appearance
of Native Americans. Putting beads, a buffalo robe and a feather headdress
on Mao Zedong would never make him look like a Plains Indian. Although the
Chinese and their morphological relatives are the dominant presence in eastern
Asia today, they are not the only people who live there, and there is reason
to believe that they were not present at all at the northeast edge of the
continent as the Pleistocene came to an end. The original human occupants
of that territory are represented today by only a remnant, the Ainu of Hokkaido,
the northeasternmost of the Japanese islands (fi g.1). The long and high-bridged
nose, the lack of epicanthic eye folds, the level upper margin of the eye
sockets and the flat cheekbones of the Ainu are more compatible with stereotypic
images of Plains Indians even if the effect is spoiled by the body hair and
full beard that is found on many adult males. Still, the presence of an
indigenous and very un-Chinese-looking people at the northeastern edge of
the Asian continent warrants further contemplation when the question of the
origins of the New World populations is being considered. g.1). The long and high-bridged
nose, the lack of epicanthic eye folds, the level upper margin of the eye
sockets and the flat cheekbones of the Ainu are more compatible with stereotypic
images of Plains Indians even if the effect is spoiled by the body hair and
full beard that is found on many adult males. Still, the presence of an
indigenous and very un-Chinese-looking people at the northeastern edge of
the Asian continent warrants further contemplation when the question of the
origins of the New World populations is being considered.
Fig.1: Ainu man and women at Piratori, on the Island of Yezo (photo: Bishop
1925, Smithsonian Ann. Rep.).
Of course, assessing whether people “look” Chinese or
“look” Native American is completely subjective no matter how much
there may be to an underlying reality being so judged. There is also the
question as to the affinities of the Ainu themselves. Their European appearance
has been remarked upon by anthropologists over the last two centuries (Desmoulins
1826:289-290; Broca 1860:481; von Baelz 1901:245; Hooton 1946:586 among others).
Genetic comparisons, however, have not confirmed the verdict of the eye (Omoto
1970, 1972; Omoto and Harada 1975). Still, the very un-Asian appearance of
the Ainu suggests that some kind of quantified test of morphological similarities
and differences should be worth pursuing. Previous attempts to do this have
yielded various results (Yamaguchi 1982; Dodo 1986; Howells 1986; Hanihara
1992, 1993, 1994; Ishida and Dodo 1993). One of the reasons for the different
pictures produced is the somewhat restricted distribution of the samples
used for comparison. When a more comprehensive set of samples representing
all of the major human population blocks in the world - as well as what can
be gleaned from Late Pleistocene samples - were all treated at once, a broader
perspective was gained (Brace et al. 2001).
Craniofacial Measurements Through Time: Before undertaking that review,
however, there is another matter that should be dealt with. That is the question
of the stability through time of a given morphological configuration. Ever
since the demonstration by Franz Boas, early in the last century, showing
how much the infamous “cephalic index” could change between one
generation and the next following the relocation of the population being
tested (Boas 1903), there has been skepticism concerning the utility of cranial
metrics in documenting continuity through time (Lewis in press). What Boas
actually demonstrated, however, was that the use of a proportion between
two adaptively trivial dimensions (i.e. areas of the skull whose shapes are
not controlled by selection) does not give a reliable indication of inherited
relationships. Both professional scholars and other interested people have
uncritically accepted the idea that substantial changes in configuration
have taken place as a matter of course over time periods amounting to only
a few thousand years.
The idea has been promoted that not only the bodily proportions but also
the proportions of parts of the face and skull have undergone major changes
because of very recent alterations in way of life. For example, it has been
proposed that arm, body, and leg proportions and also the configuration of
the craniofacial base and the elongation of the upper respiratory tract including
the nasal skeleton in Polynesians have been the result of the impact of the
selective forces that have affected them during the exploration and settling
of Remote Oceania (Houghton 1996). The expansion of the upper respiratory
tract was said to have impinged on the developmental processes which controlled
the lower parts of the facial skeleton. That explanation was offered to account
for the fact that the nasal and associated parts of the face in Polynesians
are longer while the teeth and tooth-bearing parts are relatively reduced
when they are compared to those of the peoples of mainland Asia, the presumed
original source of the inhabitants of the Pacific. Since the Austronesian
spread only goes back little more than 4,000 years and Remote Oceania has
been settled for no more than 2,000 years (Pawley and Green 1975), this
presumably demonstrated that the differentiation of a Polynesian from an
essentially Chinese craniofacial configuration was accomplished in a matter
of just a few thousand years.
The case of the affinities of the contested prehistoric Kennewick skeleton
from the State of Washington has raised similar matters. That particular
individual (fig.2) is more than 9,000 years old according to the first reported
radiocarbon dates (reported in Science May 22, 1998, p.3), and the initial
impression of a number of observers (and I am one of them) was that its
craniofacial form does not resemble that of the living Native Americans who
wish to claim it as a direct ancestor, and who have declared their intent
to rebury it without any effort to test it metrically to 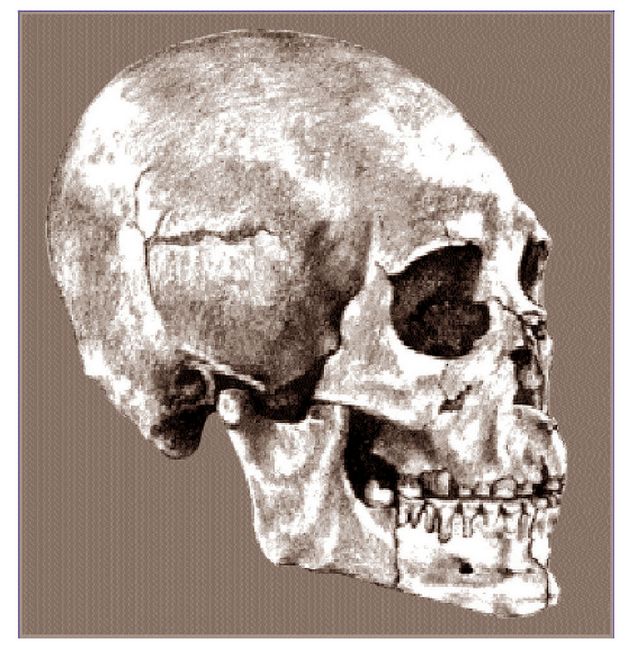 see whom it most
resembles. To those of us who have said that it does not look like the current
inhabitants of the area, the off-handed answer by representatives of the
claimants is that craniofacial form has simply changed over the last 9,000
years. In their view this is just what should be expected and does not change
what they assert to be the fact that it is their lineal ancestor (Preston
1997:74). see whom it most
resembles. To those of us who have said that it does not look like the current
inhabitants of the area, the off-handed answer by representatives of the
claimants is that craniofacial form has simply changed over the last 9,000
years. In their view this is just what should be expected and does not change
what they assert to be the fact that it is their lineal ancestor (Preston
1997:74).
Fig.2: The skull of Kennewick Man (after drawing by Chatters; Smithsonian
Arctic Studies website).
The question then needs to be addressed: how long do recognizable craniofacial
configurations last through time, and how fast can such configurations change?
The material available for testing such matters is relatively limited, often
amounting to single specimens from different time periods and widely dispersed
localities, and assertions of relationship and distinction are regularly
made without any quantitative effort to test their probabilities. A few such
tests have actually been undertaken. The famous Cro-Magnon skull (fig.3)
of approximately 28,000 years ago (White 1989:93) has been compared using
multiple discriminant functions (see glossary) to representatives of the
major groups of the living populations of the world. The figures initially
produced were posterior probabilities which can tell what populations the
specimen being tested can be excluded from, but that are less certain as
indicators of the populations to which the specimen actually will belong. What was demonstrated was that a configuration like that of Cro-Magnon could
not be found in any of the populations of the world except those at the northwest
edge of Europe (Brace 1991:189). Evidently a Cro-Magnon sort of craniofacial
configuration has been identifiably European for nearly 30,000 years at the
n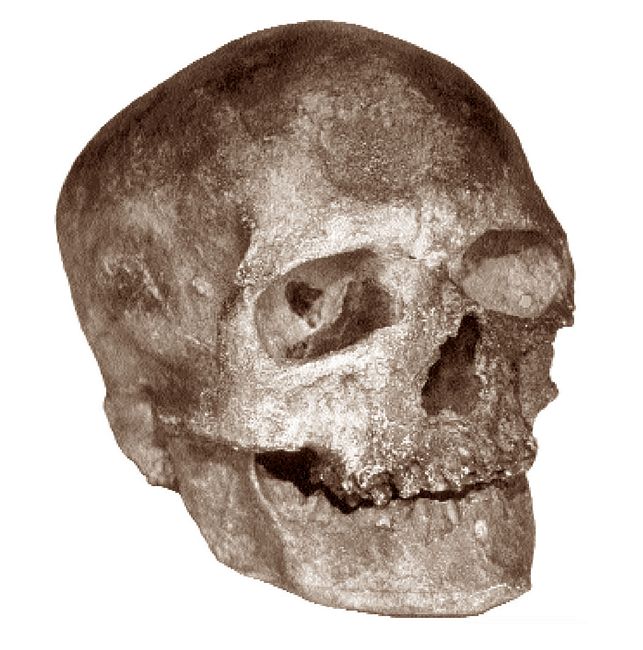 orthwest edge of human habitation. When the same statistical procedure is
used to test the affinities of the Cro-Magnon contemporary, Predmostí
3 from the Czech Republic, essentially the same conclusions were confirmed
(Brace 1991:189). That is, the strongest affinities were with the living
people of northwest Europe although there were weaker ties with the prehistoric
inhabitants of Japan and with the living Polynesians and Native Americans
along the US-Canada border. As will be shown later, the latter ties are
notirrelevant hints. orthwest edge of human habitation. When the same statistical procedure is
used to test the affinities of the Cro-Magnon contemporary, Predmostí
3 from the Czech Republic, essentially the same conclusions were confirmed
(Brace 1991:189). That is, the strongest affinities were with the living
people of northwest Europe although there were weaker ties with the prehistoric
inhabitants of Japan and with the living Polynesians and Native Americans
along the US-Canada border. As will be shown later, the latter ties are
notirrelevant hints.
Fig.3: Skull from Cro-Magnon, France (photo: Athena Review, from cast at
the American Museum of Natural History). Interestingly enough, when the Italian Neanderthal from Monte Circeo of between
51,000 and 57,000 years ago (Schwarcz et al. 1991:316) is tested in the same
fashion, it also shows that it is convincingly distinguished from all the
living populations of the world except those at the northwest edge of Europe
(Brace 1991:189). When European Neanderthals are treated as a group and compared
with the living populations of the world using a neighbor-joining clustering
procedure (see glossary), they always show a link to Europe before they tie
in with any other population (Brace et al. 2001:10018). Evidently, despite
the century-long insistence that Neanderthals are radically distinct from
“anatomically modern” Homo sapiens, the European Neanderthals not
only share something with living humans but the form shared is with the living
humans who come from the same part of the world that the Neanderthals inhabited
over 50,000 years ago.
For some time, now, the claim has been promoted that the more than
90,000-year-old fossils from Jebel Qafza in Israel can be called
“Proto-Cro-Magnon” (Vandermeersch 1981:9; Schwarcz et al. 1988;
Valladas et al. 1988). When Qafza 6 was tested by the multiple discriminant
function procedure against the living populations of the world, the probability
of its proportions occurring in a European population was 0.000. It was least
likely to be distinguished from a sample of West Africans to which were added
the Haya from Tanzania (0.662). When typicality probability levels were used
to test the differences between Qafza 6 plus Qafza 9 and various African
groups as well as with Cro-Magnon 1, the figures for the comparisons with
Africans averaged around 0.5 while for Cro-Magnon they were 0.001 (Quintyn
1999:228-229). Evidently it is easier to get a “morphologically
modern” European out of a Neanderthal predecessor than out of Qafza
while it is easier to get a “morphologically modern” West African
from Qafza than from a Neanderthal. The human remains at Qafza were associated
with a fauna that represented an incursion into the Middle East from sub-Saharan
Africa (Tchernov 1992:176). There is reason to suggest, then, that the Qafza
people were immigrants from sub-Saharan Africa. If that can be considered
the case, then Qafza represented contemporary sub-Saharan Africans. Continuity
in craniofacial form in Africa, then, can be shown to have persisted for
more than 90,000 years (i.e. supporting the idea that Paleoindian, or
Kennewick-like skull traits could have persisted 9,000 years).
Interestingly enough, the dental metrics tell the same story. Summary
tooth size is 1503 mm2 for Qafza and 1281 mm2 for the living Ashanti
(Vandermeersch 1981: 176; Brace et al. 1991). The figure for the
“classic” Neanderthals is 1415 mm2 and for living Europeans is
1127 mm2 (Brace 1979). Although there is more than a 200 mm2 reduction in
summary tooth size between Qafza and living Africans and between Neanderthals
and living Europeans, the tooth size profile from I1 (the upper central incisor)
to M3 (the upper third molar) is parallel for the Qafza/African comparison
and also for the Neanderthal/ European comparison. The Qafza/European comparison
and the Neanderthal/African comparison show that those profiles are quite
different (Brace 1996). Evidently a reduction of all the teeth to proportionally
the same extent would produce a modern European dental arch out of a Neanderthal
one just as it would produce a modern African dental arch out of the condition
represented by Qafza. A similar proportional reduction would not produce
the European condition out of Qafza. In addition, the total quantity of reduction
needed to produce a modern European dentition out of a Qafza-sized predecessor
is greater than would be the case if one postulated a Neanderthal-sized
predecessor. Qafza then is an unlikely candidate as a
“Proto-Cro-Magnon” or the ancestor of modern Europeans. The realization
that the indigenous Mousterian cultural traditions of Europe from the Atlantic
coast all the way to Eastern Europe were the most likely sources of the
succeeding Upper Paleolithic cultures makes it vanishingly unlikely that
the bearers of those cultures were invaders from elsewhere (Svoboda 1986:240;
Clark and Lindly 1989:640; Churchill and Smith 2000).
The dental and craniofacial pattern comparison of Qafza with living Africans
produces metric similarities and differences that are of exactly the same
order of magnitude as the comparison of Neanderthals to living Europeans.
Qafza, however, is accepted as “modern” in form while the Neanderthals
are referred to as “archaic” and therefore not acceptable as possible
European ancestors. This, however, is simply a subjective stance, and it
contains within it a presumption that evolution could not have occurred.
When one considers all the aspects of the Qafza skeletal remains vis-a-vis
modern Africans, it is clear that Qafza is every bit as archaic as
are the European Neanderthals vis-a-vis living Europeans (Quintyn 1999).
The final reason why Neanderthals were dismissed as possible ancestors of
Europeans is the assumption that the change from Neanderthal to
“modern” form was too abrupt to have happened by in situ evolution
(Tattersall 2000:61). As one report put it, “Neanderthals were replaced
by modern humans in Europe within too short a period for the former to have
evolved into the latter” (McBrearty and Brooks 2000:454).
As with the argument that Neanderthal form was just too different to serve
as a source for modern humans, the claim that there just was not enough time
for the change to take place has just as little basis in demonstrable fact.
The common assumption that the Neanderthals were replaced in relatively
categorical fashion is based on the visual image conjured up when a fully
developed Neanderthal of over 50,000 years ago is juxtaposed with the skull
of the “Old Man” of Cro-Magnon of half that age or even with the
skull of a living human being. A nice quantitative index of the change from
the Neanderthal to the “modern” condition can be seen in the record
of tooth size starting with the early Neanderthals from Krapina in Croatia
130,000 years ago (Rink et al. 1995) and proceeding via the “classic”
and Late Neanderthals, the Early and Late Upper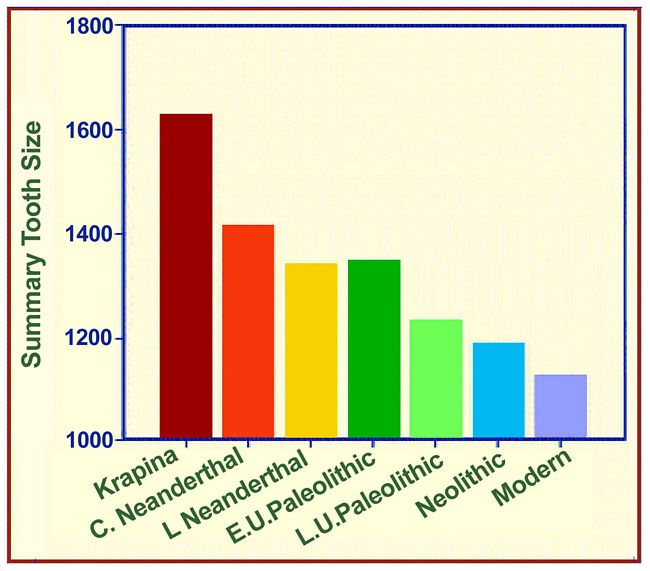 Paleolithic, the Neolithic,
and the living in Europe (Brace et al. 1987). Paleolithic, the Neolithic,
and the living in Europe (Brace et al. 1987).
Fig.4: Summary tooth size figures in mm2 for Early Neanderthals represented
by Krapina in Croatia, “Classic” Neanderthals, Late Neanderthals
represented by Hortus in France, Early Upper Paleolithic represented by
Predmostí in the Czech Republic, Late Upper Paleolithic (Prayer 1976),
and the European Neolithic (Brace 1979). This is depicted in Figure
4 where the vertical axis is Summary Tooth Size or TS, the sum total of the
cross-sectional areas of each tooth category both upper and lower. As can
be seen in Figure 4, the big jump is not between Neanderthal and modern but
between the early Neanderthals at Krapina in Croatia and the
“Classic” Neanderthals of Western Europe dating to around 50,000
years ago. The time gap between Krapina and the “Classic” Neanderthals
is also the largest interval. The difference between the “Classic”
Neanderthals and the Late Neanderthal/Early Upper Paleolithic is not so great
as the difference between the Early and the Late Upper Paleolithic. When
the regression of tooth size through time is plotted, dental reduction from
Krapina to the end of the Pleistocene was proceeding at the rate of 1% every
2,000 years. Starting with the Neolithic, however, the rate doubled to become
1% every 1,000 years (Brace et al. 1987).
In any case, the business of converting a Neanderthal head into one of modern
form involved a significant reduction in the size of the teeth and of course
the jaws that held them (fig.5). The overlap at the end of what is called
Neanderthal and the beginning of “modern” is so complete
that they are essentially the same. The earliest “moderns,” then,
are really half way in between Neanderthals and the l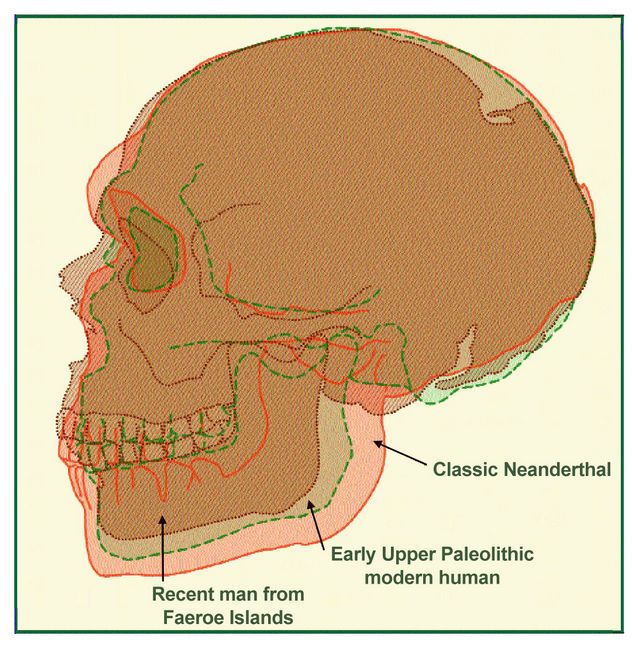 iving in both form and
time. This can be visually represented by equating nasion-opisthocranion
length (see box 1) and comparing the rest of the craniofacial outline as
shown in Figure 5. The specimens compared are La Ferrassie I, Predmostí
3, and a recent male from the Faeroe Islands. The cranial outline is essentially
similar, but what changes is the face, and it is largely accomplished by
the reduction of the jaws and the tooth-bearing part of the facial skeleton.
The brow ridge at the top of the face also undergoes a reduction that keeps
in step with the reduction of the teeth and jaws. iving in both form and
time. This can be visually represented by equating nasion-opisthocranion
length (see box 1) and comparing the rest of the craniofacial outline as
shown in Figure 5. The specimens compared are La Ferrassie I, Predmostí
3, and a recent male from the Faeroe Islands. The cranial outline is essentially
similar, but what changes is the face, and it is largely accomplished by
the reduction of the jaws and the tooth-bearing part of the facial skeleton.
The brow ridge at the top of the face also undergoes a reduction that keeps
in step with the reduction of the teeth and jaws.
Fig.5: Superimposed cranial outlines of a Classic Neanderthal (La Ferrassie
A), Early Upper Paleolithic “modern” (Predmostí 3), and
recently living male from the Faeroe Islands (Drawn by Kay Clahassey from
photographs taken by the author; with permission of Dr. Jean-Louis Heim of
the Musée de l’Homme in Paris and Dr. Pia Bennike at the Panum
Institute, Copenhagen). Figures 4 and 5 provide
graphic support for the interpretation of the Predmostí remains offered
by Hrdlicka early in the last century: “The writer has seen this collection
on two occasions and he regards it as by far the most important assemblage
of material from the transitional period between earlier and the latest
Paleolithic forms. It represents in a measure the much searched-for bridge
between the Neanderthal and recent man” (Hrdlicka 1914:551).
The persistent refusal to consider Neanderthals as representatives of a previous
stage in human evolution is mainly a survival of anti-evolutionary thinking
in paleoanthropology. In many respects, the mind-set of the field has never
quite caught up with the establishment of evolutionary biology in the 20th
century, and it has a way to go before it catches up with square one in the
21st.
The Shaping and Spread of “Modern” Human Form: The mechanism
producing the reductions both in the facial and the postcranial skeleton
have been discussed elsewhere and will only be mentioned briefly here (Brace
1995, 2000). In both instances, the reductions were the consequences of the
relaxation of selective force pressures which followed innovations in the
cultural realm. The development of effective projectiles represented by the
invention of Levallois points in the African Acheulean over 240,000 years
ago (McBrearty et al. 1996) improved the hunting capabilities of Middle
Pleistocene hominids and reduced some of the necessity for dealing with prey
animals through sheer brute strength (Shea 1995; Boeda et al. 1999). Charles
Darwin was the first to articulate the idea that the relaxation of selective
forces maintaining a particular structure was followed in time by the reduction
of the structure itself (Darwin 1859:134-139, 454).
Darwin, of course, did not know what a gene was and how it worked. The full
realization of that had to await the birth of molecular genetics nearly a
century after his seminal work was published. It is now evident that random
changes in the basic genetic material have non-random consequences, most
of which are detrimental to the development of a trait if that trait is necessary
for survival. If circumstances change, however, and the trait is no longer
necessary, i.e. if it becomes neutral, then chance mutations affecting its
form will not be selected against. Most mutations interfere with the development
of the trait to which they contribute, and, if they are allowed to accumulate,
will lead to the failure of that trait to develop to the full extent that
it had done during the period when it had significant survival value. The
phenomenon has been called the Probable Mutation Effect, which states that
the most likely effect of the most likely mutation is structural reduction
(Brace 1963, 2000). This is the mechanism that accounts for those reductions
observed by Darwin in instances where selective force relaxation had occurred.
In humans, the development of effective hafted projectile points took away
some of the selective force favoring the maintenance of postcranial robustness
and muscularity. Even more effective, however, was the significance of the
discovery and application of string (Brace 1995:272-273). With that initially
“unobvious” innovation, it was possible to devise nooses for snares
that allowed the capture of prey with no major effort on the part of the
hunter. A hook attached to the end of a string gave access to denizens of
the aquatic world again with the expenditure of relatively little effort.
Finally the use of string to construct netting gave the hunter access to
fish and birds in great quantities and made available an enormous biomass
that could not be tapped by earlier hunters. Again this could be gained with
the expenditure of relatively little effort. The reduction in skeletal and
muscular robustness that becomes obvious in the Late Neanderthals just continues
in the early “moderns” of the initial Upper Paleolithic and goes
on to the present day.
The primary use of the teeth is in the processing of food. Non-dental food
processing practices reduced the amount of previously necessary chewing and
relaxed the selective pressures that had kept tooth size at approximately
the same level since the beginning of the Pleistocene nearly two million
years ago. Habitation in the North Temperate Zone was made possible after
the Middle Pleistocene by the use of fire, not only for warmth but also for
thawing meat that had frozen before it could be consumed. Fire used for food
preparation - cooking - reduced the amount of mandatory mastication, and
led to the reduction in to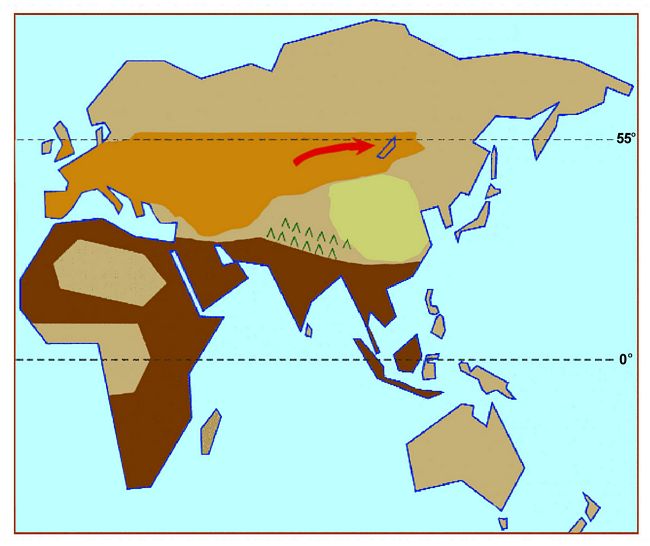 oth and jaw size that converted Neanderthal into
“modern” face form (Brace 1979, 1995, 2000; Brace et al. 1987,
1991). It is no accident that the greatest average manifestation of dental
reduction from Middle Pleistocene levels is found in just those populations
who have longest resided in the North Temperate Zone. oth and jaw size that converted Neanderthal into
“modern” face form (Brace 1979, 1995, 2000; Brace et al. 1987,
1991). It is no accident that the greatest average manifestation of dental
reduction from Middle Pleistocene levels is found in just those populations
who have longest resided in the North Temperate Zone.
Fig.6: Course of the eastward spread of Levallois tool-making people about
200,000 years ago (after Brace).
Starting late in the Middle Pleistocene, the Levallois technique diffused
rapidly to the occupied parts of the western and northern sections of the
Old World (Schick 1998), but it was not adopted in much of the Indian
subcontinent or farther east in China, Korea, Japan or Southeast Asia (Rajendran
et al. 1977-1978; Matsufuji 2000). Initially it was evidently adopted by
the people who were already living in the Temperate Zone, but its spread
along the northern edge of human habitation and eastwards at the level of
the 55th parallel towards Lake Baikal in Kazakhstan well over 200,000 years
ago (fig.6) (Derev’anko 1998:342) had to be the result of the movement
of people who were entering territory that had not previously been occupied.
Even though the evidence is restricted to one juvenile lower second molar
and a permanent upper central incisor (Shapkova and Derev’anko 2000:129),
it is not inconsistent with the idea that the people who moved into that
area bringing the Levallois industry with them were Neanderthals of the same
kind as those making Levallois tools further to the west.
Now since their subsistence and food-processing practices were the same as
those of their relatives farther west, and if it were those practices that
led to the transformation of Neanderthal into “modern” form in
Europe, the same processes should have led to the transformation of those
eastern Neanderthals into “modern” form as well. Furthermore, that
“modern” form should bear a stronger relationship to European
morphology than to the characteristic morphology that we associate with the
indigenous peoples of other parts of the world such as Africa, Australia
 and the core of mainland East Asia. This also has to be taken into account
when we consider the nature of the people who served as the source for the
first migrants into the New World. and the core of mainland East Asia. This also has to be taken into account
when we consider the nature of the people who served as the source for the
first migrants into the New World.
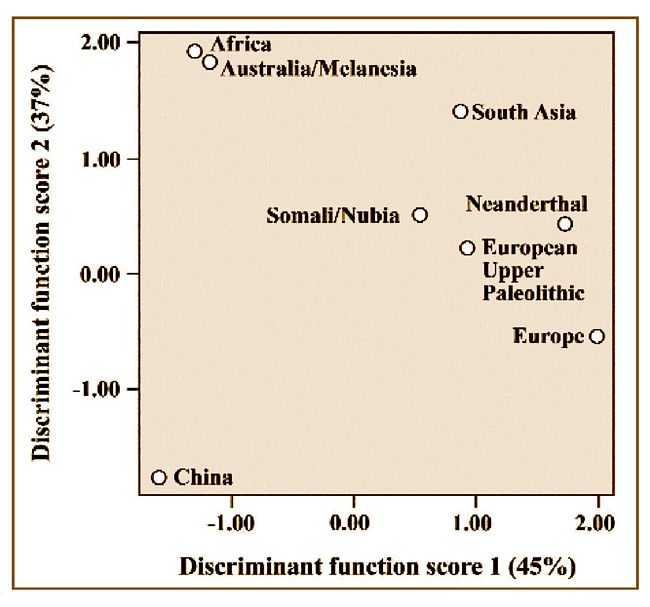
Fig.7: A plot of the first two canonical discriminant function scores for
samples representing the main regions of the Old World plus European Neanderthals
and Upper Paleolithic representatives. Just over 90% of common variance is
accounted for in the two discriminant functions plotted (after Brace et al.
2001, Figure 1 B).
Representatives of the major divergent population blocks of the Old World
can be compared with each other and with European Neanderthal and Upper
Paleolithic samples using canonical discriminant function scores. These were
generated from a battery of 21 craniofacial measurements made on samples
of each of the groups tested. The measurements used and the sample sizes
are specified in more detail elsewhere (Brace et al. 2001; see box 1). A
comparison based on the first two discriminant functions is depicted in Figure
7. Africa and Australia are quite close together while China and Europe are
about as far away from them and from each other as it is possible to be.
The Neanderthal sample is located not far from the Upper Paleolithic and
modern Europeans. When a third discriminant function is added (representing
less than 10% of the total variance), the Neanderthals are separated from
the rest of the other samples by a very considerable gap.
Removing the Neanderthals and adding more representatives from East Asia
including Mongols, Southeast Asians, the Ainu from northern Japan, plus a
sample made up of the 29,000-year-old Upper Cave of Zhoukoudian in China
and the 18,000 year-old Minatogawa specimens from Okinawa, a neighbor-joining dendrogram produces the relationships in Figure 8. 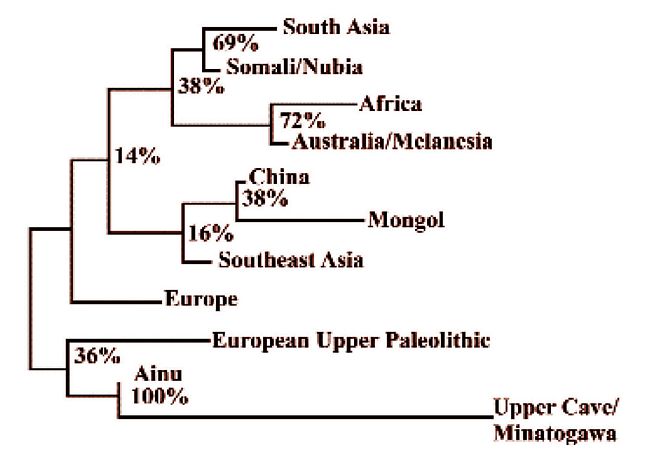 Fig.8: A neighbor-joining tree based on 1,000 bootstrap samplings showing similarities and differences between various modern and European Upper
Paleolithic groups compared in fig.12, to which are added Late Pleistocene
Asian (Upper Cave/Minatogawa) samples plus three more Asian samples. Because
the Asian prehistoric specimens were all males, the representatives of the
other samples were also restricted to males only (after Brace et al. 2001;
Fig. 2).
Fig.8: A neighbor-joining tree based on 1,000 bootstrap samplings showing similarities and differences between various modern and European Upper
Paleolithic groups compared in fig.12, to which are added Late Pleistocene
Asian (Upper Cave/Minatogawa) samples plus three more Asian samples. Because
the Asian prehistoric specimens were all males, the representatives of the
other samples were also restricted to males only (after Brace et al. 2001;
Fig. 2).
Previous tests had shown
that the Upper Cave and Minatogawa specimens behaved in similar fashion when
compared with the other populations of the world which is why they were lumped
for comparison here (Brace 1991:453). The Upper Cave and Minatogawa sample
ties to the Ainu and then to the European Upper Paleolithic before these
all link to m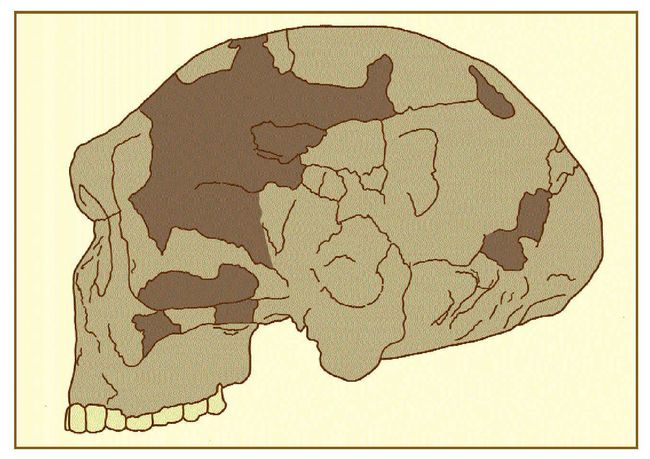 odern Europeans. This is what would be expected if the Jomon
and their relatives were indeed descendants of a stratum of related peoples
who extended across the northern edge of the Old World from Europe to Siberia
in the latter part of the Middle Pleistocene. odern Europeans. This is what would be expected if the Jomon
and their relatives were indeed descendants of a stratum of related peoples
who extended across the northern edge of the Old World from Europe to Siberia
in the latter part of the Middle Pleistocene.
Fig.9: A Chinese Neanderthal from Jinniushan. (Drawn by Kay Clahassey
from Lü 1990; reproduced as Fig. 13-12 in Brace 1995). If that stratum had been characterized by a Neanderthal degree of robustness,
then it should be legitimate to postulate an ancestor of the aboriginal
populations of the northeast edge of Asia that looked like European Neanderthals.
The 1984 discovery of a more than 200,000-year-old skeleton at Jinniushan
in Liaoning Province some 400 km northeast of Beijing fits that description
adequately (fig.9; Lü 1990; Chen et al. 1994). Following up on that
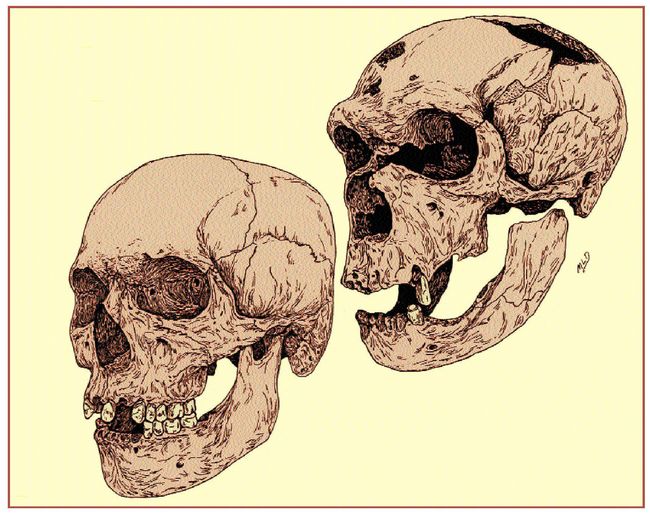
cue, Figure 10 shows a view of the French Neanderthal from La Chapelle-aux-Saints
juxtaposed with a recent Ainu from Hokkaido in the north of Japan. There
is no reason why a reduction in the size of the jaws and teeth of the same
order of magnitude as that depicted in Figure 4 could not have produced the
Ainu configuration out of a Neanderthal of that appearance over the course
of 50,000 years.
 Now if the other more distant populations such as Africa, South Asia and
Australia are removed, and Polynesians and the prehistoric Jomon from Japan
are added, we get the picture shown in Figure 12. The Polynesians are a step
closer to mainland Asia than are the Ainu, and the latter are a step closer
than are the Jomon - the obvious ancestors of the Ainu of Japan. One can
read Figure 12 as an indicator of the increasing contribution of an indigenous
Asian population to the genetic makeup of its neighbors as a result of growth
and expansion made possible by agriculture of Chinese origin within the last
10,000 years.
Now if the other more distant populations such as Africa, South Asia and
Australia are removed, and Polynesians and the prehistoric Jomon from Japan
are added, we get the picture shown in Figure 12. The Polynesians are a step
closer to mainland Asia than are the Ainu, and the latter are a step closer
than are the Jomon - the obvious ancestors of the Ainu of Japan. One can
read Figure 12 as an indicator of the increasing contribution of an indigenous
Asian population to the genetic makeup of its neighbors as a result of growth
and expansion made possible by agriculture of Chinese origin within the last
10,000 years.
Fig.10: The French Neanderthal, La Chapelle-aux-Saints, upper right,
and a recent Ainu, lower left. (Drawn by Mary L. Brace with the permission
of Jean-Louis Heim at the Musee de l’Homme in Paris, and of Professor
Takeru Akazawa of the University Museum, University of Tokyo).
The Jomon used here are made up of both Middle (5,000 years old) and Late
(3,000 years old) Jomon individuals (Tsukada 1986). Gene flow from mainland
Asia can be expected to have had an increasing impact as time went on. The
Ainu would have been more affected than the Jomon which would account for
their slightly closer link to the mainland Asian groups in Figure 11. If
the Polynesians had their roots in the Japanese archipelago as has been suggested
(Brace et al. 1989), their movement via Taiwan and the Philippines approximately
4,000 years ago would have brought them into contact with agricultural
populations of mainland origin, and this would account for the slightly closer
link shown in Figure 11.
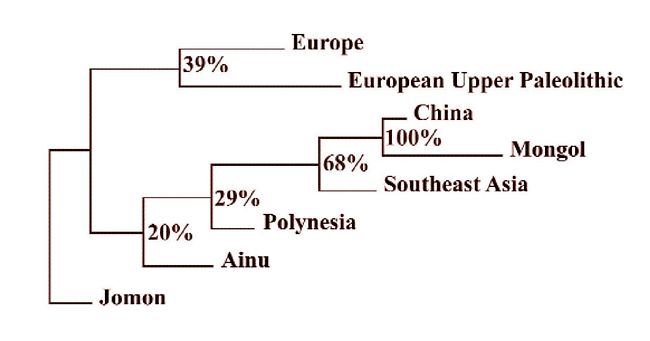 Fig.11: Neighbor-joining dendrogram of prehistoric and recent groups representing
the northern edge of the Old World from Europe to Japan, including SE Asia
and Polynesia. The pattern displayed was produced after 1,000 bootstrap samplings
(after Fig.3 of Brace et al. 2001).
Fig.11: Neighbor-joining dendrogram of prehistoric and recent groups representing
the northern edge of the Old World from Europe to Japan, including SE Asia
and Polynesia. The pattern displayed was produced after 1,000 bootstrap samplings
(after Fig.3 of Brace et al. 2001).
The Western Hemisphere: Adding representative New World samples to
the groups used in Figure 11 and arranging them with the aid of the
neighbor-joining procedure gives us the configuration visible in Figure 12.
A Bronze Age sample from western Mongolia and a modern Japanese sample were
also added. The sample labeled United States/Canadian Border is made up of
Blackfoot plus the Juntunen ossuary from northern Michigan and the Ossossane
ossuary from Ontario northwest of Toronto. When those samples were run as
separate twigs, they were very close to each other which is why they were
combined here to make a single case. As can be seen in Figure 12, this grouping
is closer to Europe, the European Upper Paleolithic 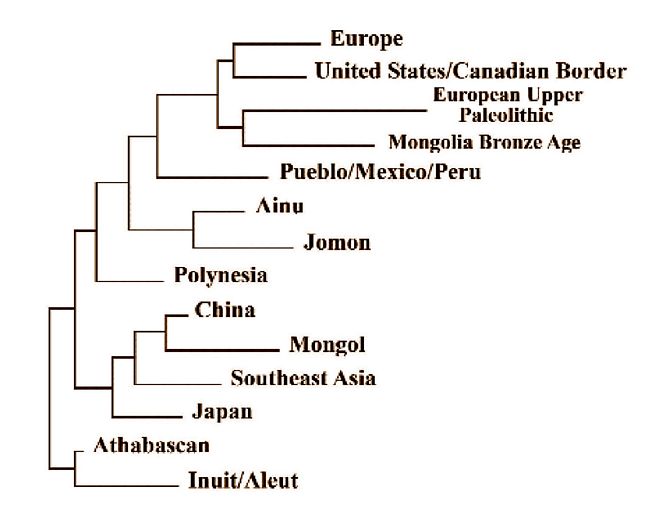 and the Mongolian Bronze
Age than to anything else. The Pueblo sample from the American Southwest
ties closely with Mexico and highland South America when run separately,
so they were combined into a single sample to run (fig.13). The Pueblo/
Mexico/Peru sample is about equidistant between the Europe-US/Canadian Border
and the Jomo n/Ainu groups but is linked least with the Mainland Asian samples.
Fig.12: Neighbor-joining dendrogram using Fig.12 groups and a series of New
World samples plus separate Mongolian Bronze Age and Japanese samples.
The Athabascan and the combined Inuit/Aleut samples are the only New World
groups who tie more closely to the core mainland Asians than to Europeans
and their relatives. Representatives of the most recent entrants from the
northeastern edge of the Old World are part of a movement that goes back
no more than 4,000 or 5,000 years, so their ancestors had to have been affected
by the burgeoning of those who had developed an agricultural mode of subsistence
in mainland Asia. The people who did that, of course, were the Chinese. It
should be no surprise, then, that there are evident links between Chinese
morphology and that shown in the Inuit/Aleut and the Athabascans.
The earlier entrants into the New World, dating back to 15,000 years ago
and perhaps more, had spread eastwards toward Alaska at a time when there
was no Bering Strait. Because of the lower sea level in the last glaciation,
the continents simply coalesced (Hopkins ed. 1967). Immigrants could have
spread generation by generation into unoccupied territory until they had
become the occupants of a new continent without even knowing it. Since this
was long before agriculture had allowed the expansion of Chinese and
Chinese-related populations, there was much less genetic impact by the Chinese
on those who constituted the first New World entrants. The residual hint
of that northern tie running all the way from Europe eastwards and showing
up in the Great Lakes region of the United States and Canada can be seen
in the distribution of the X haplotype of mitochondrial DNA (Macaulay et
al. 1999; Schurr 2000 and pp.62-75, this issue).
The suggested route of movement of people into the New World approximately
15,000 years ago is depicted by the solid arrows in Figure 13. Subsequent
population movements about 6,000 years ago were set in motion by the population
expansion made possible by the development of agriculture as well as by the
technological and resource utilization that derived from that even in areas
where agriculture was not possible. The dotted arrows show the movement of
wheat cultivating people westwards from the Middle East both north and south
of the Mediterranean and towards the Indian subcontinent. They also show
population movement from China down into Southeast Asia by rice cultivating
people with a later incursion into Korea and Japan.
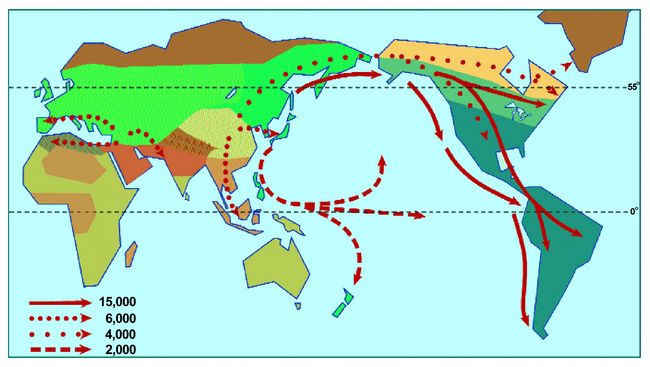
Fig.13: The solid arrows indicate the likely route of initial entry into
the New World approximately 15,000 years ago. The dotted arrows indicate
population movements initially driven by the effects of the development of
agriculture and principally occurring 5,000 years ago and less (after Brace
et al. 2001).
While rice could not be cultivated in Siberia and over into Beringia, the
techniques of extracting nourishment from all sorts of unlikely plant resources
that were ultimately derived from the knowledge gained by those who had
originally developed Asian agriculture allowed people to spread into parts
of the north that were not being exploited by those concentrating on hunting.
The mind-boggling ingenuity shown in the use of those unlikely resources
is abundantly demonstrated in one of the more remarkable anthropological
treatises, namely Franz Boas’ chapter, “Recipes,” in his monograph
on the Kwakiutl of the American Northwest Coast (Boas 1921). The course of
the movement within the last 5,000 years of people sustaining themselves
by various kinds of specialized resource-utilization ingenuity is depicted
by the dotted arrows in Figure 13 with larger spaces between the dots. Obviously
the coastal people at the northern edge of that distribution had focused
their ingenuity on marine rather than plant resources since nothing much
grows on land there.
The last movement depicted in Figure 13 is the spread of marine resource-using
specialists latterly coupled with root-crop agriculture out into the Pacific.
This is indicated by the dashed-line arrows. The beginnings from the Japanese
archipelago some 4,000 years ago led to the designation of these people as
members of what was called the “Jomon-Pacific” cluster (Brace et
al. 1989:105). The final movement out into “Remote Oceania,” as
with the Na-Denè movement down into the American Southwest, only happened
within the last 2,000 years.
Conclusion: From the morphological analysis presented here as well
as from the molecular genetic evidence, it seems only appropriate that the
first entrants into the New World should properly be referred to as Eurasians.
Although the second evident wave of immigrants, the Aleut/Inuit and the
Athabascans, show a much stronger connection to the living populations of
mainland Asia, the presence of an obvious residuum of European form fully
qualifies them also to be called Eurasian. If it was the legacy of gracilized
northern Neanderthals that contributed to the significant difference between
the original human populations of the New World and the post-Pleistocene
entrants, the question still remains concerning the form of the antecedents
of the modern inhabitants of the Asian mainland and their relatives. At present,
however, the almost complete absence of hominid fossils from the Pleistocene
in central and southern China means that we have no way of dealing with such
a question. The archaeological record shows that people were there (Clark
and Schick 1988), but the one specimen that is well enough preserved to give
a hint as to morphology, the Dali skull found not far from Xian and over
200,000 years old (Chen and Yuan 1988), has a shape that recalls Homo erectus
more than Homo sapiens. As a consequence, it is not of much help in suggesting
the course of the emergence of modern Asian craniofacial form. The solution
to the problem will only come with discoveries yet to be made. .
, Acknowledgements: Support for the work on which this report is based
was provided by the Committee on Scholarly Communication with the People’s
Republic of China in 1980 and 1985; by the L. S. B. Leakey Foundation in
1986; by the National Science Foundation BNA- 9616298 in 1986-1988, and
INT-9107991 in 1992-1993; and by the University of Michigan Museum of
Anthropology Research Fund in 1986, 1990-1992. Efforts to incorporate added
material from European and Russian Paleolithic and Mesolithic specimens and
from the Brazilian Lagoa Santa material in Copenhagen were denied support
by the National Science Foundation in 1992, 2000-2001, and by the L. S. B.
Leakey Foundation and by the Wenner-Gren Foundation for Anthropological Research
in 2001-2002. Crucial craniofacial measurements were contributed for Mongolian
and Native American samples by A. Russell Nelson of the Department of
Anthropology at the University of Wyoming and by Pan Qifeng of the Institute
of Archaeology in the Chinese Academy of Social Sciences in Beijing. Essential
computer assistance was provided by Noriko Seguchi of the Museum of Anthropology
at the University of Michigan.
References:
Acosta, J. Orig. 1589. The Natural & Moral History of the Indies. Reprinted,
1880, fromthe English translation of Edward Grimestone, 1604. 2 vols. London,
Hakluyt Society.
Baelz, E. 1901. “Menschen-Rassen Ost-Asiens mit specieller Rücksicht
auf Japan.” Zeitschrift für Ethnologie 33:166-89, 202-7, 214, 216,
217, 245-9, 393-4.
Boas, Franz. 1903. “Heredity in head form.” American Anthropologist
5(3):530-538. 1921.
Boas, Franz. 1913. “Recipes” in The Ethnology of the Kwakiutl Based
on Data Collected by George Hunt. pp. 305-601. Thirty-Fifth Annual Report
of the Bureau of American Ethnology to the Secretary of the Smithsonian Instit.
1913-14. Washington DC, Gov’t Printing Office.
Boeda, E., J.M. Geneste, C. Griggio, N. Mercier, S. Muhesen, J. L. Reyss,
A. Taha and H. Valladas. 1999. “A Levallois point imbedded in the vertebra
of a wild ass (Equus africanus): Hafting, projectile, Mousterian hunting
weapon.” Antiquity 73:394- 402.
Brace, C. Loring. 1963. “Structural reduction in evolution.” The
American Naturalist 97:39-49.
Brace, C.L. 1979. “Krapina, ‘classic’ Neanderthals, and the
evolution of the European face.” Journal of Human Evolution 8(5):527-550.
Brace, C.L.1991. “Monte Circeo, Neanderthals, and continuity in European
cranial morphology: A rear end view.” In The Circeo I Neandertal
Skull, Studies and Documentation. M. Piperno and G. Scichilone (eds.),
pp.175-195. Roma, Museo Nazionale Preistorico Etnografico “Luigi
Pigorini,” Istituto Poligrafico e Zecca Dello Stata, Libreria Dello
Stato.
Brace, C.L. 1995. The Stages of Human Evolution. 5th ed. Englewood-Cliffs,
New Jersey, Prentice-Hall.
Brace, C.L. 1996. “Cro-Magnon and Qafzeh: Vive la difference.”
Dental Anthropology Newsletter 10(3):2-9.
Brace, C.L. 2000. Evolution in an Anthropological View. Walnut Creek, California,
AltaMira Press.
Brace, C.L., M.L. Brace and W.R. Leonard. 1989. “Reflections on the
face of Japan: A multivariate cranifacial and odontometric perspective.”
Amer. J. of Physical Anthropology 78(1):93-113.
Brace, C.L., A.R. Nelson, N. Seguchi, H. Oe, L. Sering, P. Qifeng, L. Yongyi,
and kD. Tumen. 2001. “Old World sources of the first New World human
inhabitants.” Proc. National Academy of Sciences 98(17):10017-10022.
Brace, C.L., K. Rosenberg and K.D. Hunt. 1987. “Gradual change in human
tooth size in the late Pleistocene and post-Pleistocene.” Evolution
41(4):705- 720.
Brace, C.L., S.L. Smith and K.D. Hunt. 1991. “What big teeth you had,
Grandma! Human tooth size, past and present.” In M.A. Kelley and C.S.
Larsen (eds.) Advances in Dental Anthropology. pp.33-57. New York, Wiley-Liss.
Brace, C.L., and D. Tracer. 1992. “Craniofacial continuity and change:
A comparison of Late Pleistocene and recent Europe and Asia.” In T.
Akazawa, K. Aoki and T. Kumura (eds.) The Evolution and Dispersal of Modern
Humans in Asia. pp.439-471. Tokyo, Hokusen-Sha Publishing Co.
Broca, Paul. 1860. “Recherches sur l‘hybridité animate en
générale, considérés dans leurs rapports avec
le question de la pluralité des espèces humaines.” Paris,
J. Claye. pp.433-664.
Chen Tiemei, and Yang Sixun. 1988. “Uranium-series dating of bones and
teeth from Chinese Palaeolithic sites.” Archaeometry 30(1):59-76.
Chen Tiemei, Yang Quan and Wu En. 1994. “Antiquity of Homo sapiens in
China.” Nature 368:55-56.
Churchill, S., and F. Smith. 2000. “Makers of the Early Aurignacian
of Europe.” Yearbook of Phys. Anth. 43:61-115.
Clark, G.A., and J.M. Lindly. 1989. “The case for continuity: Observations
on the biocultural transition in Europe and western Asia.” In P. Mellars
and C. Stringer (eds.) The Human Revolution: Behavioural and Biological
Perspectives on the Origins of Modern Humans. pp.626-676. Edinburgh, Edinburgh
Univ. Press.
Clark, J., and K. Schick. 1988. “Context and content: impressions of
Palaeolithic sites and assemblages in the People’s Republic of China.”
J. of Human Evolution 17(4):439-448.
Darwin, C.R. 1859. On the Origin of Species by Means of Natural Selection,
or the Preservation of Favoured Races in the Struggle for Life. London, John
Murray.
Derev’anko, A. 1998. “Human occupation of nearby regions and the
role of population movements in the paleolithic of Siberia.” In A.
Derev’anko, D. Shimkin and W.R. Powers (eds.) The Paleolithic of Siberia:
New Discoveries and Interpretations. pp.336-351. Urbana and Chicago, Univ.
Illinois Press.
Desmoulins, A. 1826. Histoire naturelle des races humaines d‘après
des recherches speciales d‘antiquités, de physiologic,
d‘anatomie et de zoologie etc. etc. Paris, Mequignon-Marvis.
Dodo, Y. 1986. “Metrical and non-metrical analyses of Jomon crania from
eastern Japan.” In T. Akazawa and C. Aikens (eds.) Prehistoric
Hunter-Gatherers in Japan. pp.137-61. Tokyo, Univ. of Tokyo, Bulletin No.
27 of the Univ. Museum.
Frayer, D.W. 1976. Evolutionary Dental Changes in Upper Paleolithic and
Mesolithic Human Populations. Doctoral Dissertation (Anth.). Ann Arbor, Univ.
of Michigan.
Hanihara, T. 1992. “Dental and cranial evidence on the affinities of
the east Asian and Pacific populations.” In K. Hanihara (ed.) Japanese
as a Member of the Asian and Pacific Populations. pp.119-137. Kyoto, Internat.
Symposium 4, Internat. Research Center for Japanese Studies.
Hanihara, T. 1993. “Craniofacial features of Southeast Asians and Jomonese:
A reconsideration of their microevolution since the late Pleistocene.”
Anthropological Science 10(1):25-46.
Hanihara, T. 1994. “Craniofacial continuity and discontinuity of Far
Easterners in the Late Pleistocene and Holocene.” Journal of Human Evolution
27(5):417-441.
Hooton, E.A. 1946. Up From the Ape. 2nd ed. New York, Macmillan.
Hopkins, D.M. (ed.) 1967. The Bering Land Bridge. Stanford, California, Stanford
University Press.
Houghton, P. 1996. People of the Great Ocean: Aspects of Human Biology of
the Early Pacific. Cambridge, Cambridge Univ. Press.
Howells, W.W. 1986. “Physical anthropology of the prehistoric
Japanese.” In R. Pearson, G. Barnes, and K.L. Hutterer (eds.) Windows
on the Japanese Past: Studies in Archaeology and Prehistory. pp.85-99. Ann
Arbor, Michigan, University of Michigan Center for Japanese Studies.
Hrdlicka, A. 1914. “The most ancient skeletal remains of man.”
Annual Report of the Board of Regents of the Smithsonian Inst. pp.491-552.
Washington, DC, Government Printing Office.
Ishida, H., and Y. Dodo. 1993. “Nonmetric cranial variation and the
population affinities of the Pacific peoples.” American Journal of Physical
Anthropology 90(1):49-57.
Jefferson, T. 1787. Notes on the State of Virginia. London.
Lewis, Herbert S. in press. “The passion of Franz Boas.” American
Anthropologist.
Lü Zun-E. 1990. “La découverte de l’homme fossile de
Jing-niu-shen: Première étude.” I’Anthropologie
94(4):899-902.
Macaulay, V., M. Richards, E. Hickey, E. Vega, F. Cruciani, V. Guida, R.
Scozzari, B. Bonné-Tamir, B. Sykes and A. Torroni. 1999. “The
emerging tree of West Eurasian mtDNAs: A synthesis of control region sequences
and RFLPs.” Amer. J. of Human Genetics 64(1):232-249.
Matsutuji, K. 2000. “Appearance of early blade technique in Northeast
Asia.” Acta Anthropologica Sinica 9 (Supp.):154-157.
McBrearty, S., L. Bishop and J. Kingston. 1996. “Variability in traces
of Middle Pleistocene hominid behavior in the Kapthurian Formation, Baringo,
Kenya.” Journal of Human Evolution 30(6):563-580.
McBrearty, S., and A.S. Brooks. 2000. “The revolution that wasn’t:
A new interpretation of the origin of modern behavior.” J. of Human
Evolution 39(5):453 -563.
Omoto, K. 1970. “The distribution of polymorphic traits in the Hidaka
Ainu. I. Defective colour vision, PTC taste sensitivity and cerumen
dimorphism.” Journal of the Faculty of Science, Tokyo Univ., Section
V, 3(5):337-355.
Omoto, K., and S. Harada. 1975. “The distribution of polymorphic traits
in the Hidaka Ainu. II. Red cell enzyme and serum protein groups.” J.
of the Faculty of Science, Tokyo Univ., Section V, 4(2):171-211.
Pawley, A., and R. Green. 1975. “Dating the dispersal of the Oceanic
languages.” Oceanic Linguistics 12(1):1-68.
Preston, D. 1997. “A reporter at large: The lost man.” The New
Yorker 72(16):70-81.
Quintyn, C.B. 1999. The Morphometric Affinities of the Qafzeh and Skhul Hominids.
Doctoral Dissertation (Anthropology). Ann Arbor, Univ. of Michigan.
Rajendran, P., K.C. Malhotra and B.V. Bhanu. 1977-1978. “A note on the
Palaeolithic industry of Karadkhed (Maharashtra).” Bulletin of the Deccan
College Research Institute 37(1-4): 118-123.
Rink, W.J., H.P. Schwarcz, F.H. Smith and J. Radovcic. 1995. “ESR dating
of tooth enamel from the Neanderthal site of Krapina, Croatia.” Nature
378:24.
Schick, K.D. 1998. “A comparative perspective on Paleolithic cultural
patterns.” In T. Akazawa, K. Aoki, and O. Bar-Yosef (eds.) Neandertals
and Modern Humans in Western Asia. pp.449-460. New York, Plenum Press.
Schurr, T.G. 2000. “Mitochondrial DNA and the peopling of the New
World.” American Scientist 88(3):246-253.
Schwarcz, H., R. Gruhn, B. Vandermeersch, O. Bar-Yosef, H. Valladas and E.
Tcherno. 1988. “ESR dates for the hominid burial site of Qafzeh in
Israel.” J. of Human Evolution 17(8):733-737.
Schwarcz, H., A. Bietti, W. Buhay, M. Stiner, R. Gruhn, and E. Segre. 1991.
“On the reexamination of Grotta Guattari: Uranium-series and
electron-spin-resonance dates.” Current Anthropology 32(3):313-316.
Shapkova, E.G., and A.P. Derevianko. 2000. “The interpretation of
odontological features of Pleistocene human remains from the Altai.”
Arch., Ethnology & Anth. of Eurasia 1(1): 125-138.
Shea, J.J. 1995. “Behavioral factors affecting the production of Levallois
points in the Levantine Mousterian.” In H.L. Dibble and O. Bar-Yosef
(eds.) The Definition and Interpretation of Levallois Technology. pp.279-292.
Madison, Wisconsin, Prehistory Press, Monographs in Arch., No. 23.
Stewart, T.D. 1960. “A physical anthropologist’s view of the peopling
of the New World.” Southwestern Journal of Anthropology 16:259-273.
Stewart, T.D., and M.T. Newman. 1951. “An historical resumé of
the concept of differences in Indian types.” American Anthropologist
53(1): 199-36.
Svoboda, J. 1986. “The Homo sapiens neanderthalensis Homo sapiens sapiens
transition in Moravia. Chronological and archaeological background.”
Anthropos (Brno) 23(3):237-242.
Tattersall, I. 2000. “Once we were not alone.” Scientific American
282(1):56-62.
Tchernov, E. 1992. “Biochronology, paleoecology, and dispersal events
of hominids in southern Levant.” In T. Akazawa, K. Aoki and T. Kimura
(eds.) The Evolution and Dispersal of Modern Humans in Asia. pp.149-188.
Tokyo, Hokusen-Sha Publishing Company.
Tsukada M. 1986. “Vegetation in prehistoric Japan: The last 20,000
years.” In R. Pearson, G. Barnes and K. Hutterer (eds.) Windows on the
Japanese Past. Studies in Archaeology and Prehistory. pp.11-56. Ann Arbor,
Michigan, Univ. of Michigan Center for Japanese Studies.
Valladas, H., J. Reyss, J. Joron, G. Valladas, O. Bar-Yosef and B. Vandermeersch.
1988. “Thermoluminescence dating of Mousterian ‘Proto-Cro-
Magnon’ remains from Israel and the origin of modern man.” Nature
331:614-616.
Vandermeersch, B. 1981. Les Hommes Fossiles de Qafzeh (Israel). Paris. Centre
National de la Recherche Scientifique.
White, R. 1989. “Visual thinking in the Ice Age.” Scientific American
261(1):92-99.
Yamaguchi, B. 1962. “A review of the osteological characteristics of
the Jomon population in prehistoric Japan.” J. of the Anth. Society
of Nippon 90 (Supplement):77-90. Papers in honor of Professor Dr. Hisashi
Suzuki’s 70th birthday.
,
,
C. Loring Brace is Professor of Anthropology at the University of Michigan,
Ann Arbor, and Curator of Biological Anthropology at the University of Michigan
Museum of Anthropology. He has documented the impact of changing selective
forces and how they have shaped the course of human evolution. This has also
been extended into documenting the reasons for the biological differences
visible in the living human populations of the world. The study of the latter
has led to the realization that one cannot understand the nature of human
biological variation if one uses “race” as a starting point. He
is the author of The Stages of Human Evolution (5th ed. 1995) and Evolution
in an Anthropological View (2000).
This article appears on pp. 53-61 in Vol.3, No.2 of Athena
Review.
.
|